Now There Are Many (Stages)
 Now There Are Many (Stages) Where Before There Was One:
Now There Are Many (Stages) Where Before There Was One:
In Search of a Pathophysiological Classification of Cirrhosis
Guadalupe Garcia-Tsao,1 Scott Friedman,2 John Iredale,3 and Massimo Pinzani4
For more than a century and a half, the description of a liver as “cirrhotic” was sufficient to connote both a pathological and clinical status, and to assign the prognosis of a patient with liver disease. However, as our interventions to treat advanced liver disease have progressed (e.g., antiviral therapies), the inadequacy of a simple one-stage description for advanced fibrotic liver disease has become increasingly evident. Until recently, refining the diagnosis of cirrhosis into more than one stage hardly seemed necessary when there were no interventions available to arrest its progression. Now, however, understanding the range of potential outcomes based on the severity of cirrhosis is essential in order to predict outcomes and individualize therapy. This position paper, rather than providing clinical guidelines, attempts to catalyze a reformulation of the concept of cirrhosis from a static to a dynamic one, creating a template for further refinement of this concept in the future.
We already make the clinical distinction between compensated and decompensated cirrhosis, and are incrementally linking these clinical entities to quantitative variables such as portal pressure measurements and emerging noninvasive diagnostics. Moreover, mounting evidence suggests that cirrhosis encompasses a pathological spectrum which is neither static nor relentlessly progressive, but rather dynamic and bidirectional, at least in some patients. Thus, there is a pressing need to redefine cirrhosis in a manner that better recognizes its underlying relationship to portal hypertension and related circulatory changes, and more faithfully reflects its progression, reversibility and prognosis, ultimately linking these parameters to clinically relevant outcomes and therapeutic strategies. The Child-Pugh and Model for End-Stage Liver Disease (MELD) scores are currently deployed to define prognosis by modeling hepatic dysfunction, but do not provide direct evidence of the stage or dynamic state of cirrhosis. The need for more refined cirrhosis staging is especially germane given the increasing use of effective antiviral treatments in patients with hepatitis B virus (HBV) and hepatitis C virus (HCV) cirrhosis and the emergence of effective antifibrotic agents, wherein we must define favorable or unfavorable endpoints that correlate with a discrete clinical outcome in patients with cirrhosis.
The normal liver has only a small amount of fibrous tissue in relation to its size. As a result of continued liver injury, however, there is progressive accumulation of extracellular matrix, or scar. Although different chronic liver diseases are characterized by distinct patterns of fibrosis deposition,1 the development of cirrhosis represents a common outcome leading to similar clinical consequences that impose an increasing burden in clinical practice.
Go to:
Anatomical-Pathological Context
Cirrhosis is defined histologically as a diffuse process in which the normal anatomical lobules are replaced by architecturally abnormal nodules separated by fibrous tissue.2 Progressive histological stages have been defined in the process leading to the development of cirrhosis. Among the more common staging systems, the METAVIR scale is distinguished by four stages, with stage F0 representing lack of fibrosis; stage F1, portal fibrosis; stage F2, periportal fibrosis; stage F3, bridging fibrosis; and, finally, stage F4 representing cirrhosis.3 Similarly, the Ishak4 and Scheuer scoring systems5,6 attempt to semi-quantitatively define progressive fibrosis based on the pattern and relative amounts of scar within a liver biopsy specimen. In this context, once fibrosis reaches the final stages, the diagnosis of cirrhosis is established and the process is considered “end-stage” from a pathological perspective.
Go to:
Clinical Context
Cirrhosis has also been increasingly defined by clinical outcomes. In this context, cirrhosis is distinguished between compensated and decompensated stages, with different features, prognoses and predictors of death.7 Within the compensated stage, two subpopulations have been identified based on the absence or presence of varices, each of which confers a distinct prognosis. Decompensated cirrhosis is defined by the development of clinically evident complications of portal hypertension (ascites, variceal hemorrhage, hepatic encephalopathy) or liver insufficiency (jaundice). The decompensated stage can be subclassified further into a more severe stage defined by the development of recurrent variceal hemorrhage, refractory ascites, hyponatremia and/or hepatorenal syndrome.
Go to:
Hemodynamic Context
Portal hypertension is the earliest and most important consequence of cirrhosis and underlies most of the clinical complications of the disease. Portal hypertension results from an increased intrahepatic resistance combined with increased portal (and hepatic arterial) blood flow. The increased intrahepatic resistance is the result of architectural distortion (fibrous tissue, regenerative nodules), endothelial dysfunction leading to intrahepatic vasoconstriction, and intrahepatic vascular shunts between afferent and efferent vessels of the liver.8 In addition, cirrhosis is accompanied by extrahepatic hemodynamic abnormalities: vascular resistance in the splanchnic and systemic circulations in cirrhosis is decreased, leading to an increase in splanchnic blood flow that contributes to the maintenance of the portal hypertensive state.9 This vasodilatation is due to an increased production of nitric oxide.8 Splanchnic and systemic vasodilatation are not only responsible for increasing portal inflow and variceal enlargement, but they also initiate the hyperdynamic circulatory state of cirrhosis that leads to other major complications such as ascites. Moreover, vasodilation and increased portal flow are more extreme in patients with further decompensation of cirrhosis (i.e., refractory ascites, hyponatremia and hepatorenal syndrome).
The hepatic venous pressure gradient (HVPG), an indirect measure of portal pressure, is the best predictor of the development of varices,10 and is also a harbinger of decompensation (e.g., ascites, variceal hemorrhage and encephalopathy).11 Normal HVPG is 3–5 mmHg, whereas>10 mmHg is a threshold that identifies patients at risk of developing varices, and/or clinical decompensation. Thus, HVPG > 10 mmHg defines the presence of “clinically significant portal hypertension”. Notably, recurrent variceal hemorrhage and ascites do not occur when the HVPG is reduced to levels below 12 mmHg, and therefore this threshold is closely related to the presence of decompensating events.12–14
In decompensated cirrhosis, an HVPG>20 mmHg is an important predictor of a poor outcome in the setting of acute variceal hemorrhage.15 In addition to portal pressure, however, the systemic hemodynamic alterations of cirrhosis play an important role in the development of further decompensating events such as refractory ascites, hyponatremia and the hepatorenal syndrome. Remarkably, elevated HVPG also correlates with the risk of hepatocellular carcinoma.16
Go to:
Histological, Clinical, and Hemodynamic Correlations
As noted above, the histologic features of cirrhosis have not been traditionally linked to clinical outcomes. However, there is recent evidence indicating that both HVPG and semiquantitative features of histology do indeed predict hemodynamic and clinical features of chronic liver disease and cirrhosis. For example, progressive increases in HVPG correlate with increasing severity of liver disease (normal, chronic hepatitis, precirrhosis, and cirrhosis) both in alcoholic17 and in nonalcoholic liver disease.18 Patients with fibrosis stages 3 or 4 almost uniformly have an HVPG of ≥ 6 mmHg. In a recent study of posttrans-plant recurrent hepatitis C, fibrosis stage in liver biopsies correlated with concurrent HVPG measurements when performed 1 year after transplantation.19 Notably, a significant proportion of patients who did not have a histological diagnosis of cirrhosis had an HVPG>6 mmHg (i.e., they had portal hypertension) and, upon follow-up, HVPG was more predictive of clinical decompensation than histological fibrosis staging.
Simple histologic features may also have important prognostic implications in cirrhotic liver biopsies. For example, the thickness of fibrous septa correlates with HVPG and is an independent predictor of both clinically significant portal hypertension (i.e., HVPG>10 mmHg)20 and clinical decompensation.21 Moreover, digital image analysis of septal thickness, but not total fibrosis area, predicts cirrhosis decompensation.22
Go to:
Biological Context
Studies performed in the past two decades have identified several attractive targets for antifibrotic treatment. These include the major cellular sources of scar, most notably activated hepatic stellate cells and portal myofibroblasts, as well as key cytokines such as platelet-derived growth factor and transforming growth factor beta.23 The roles of bone marrow–derived cells and those arising from epithelial-mesenchymal transition are still under evaluation, but it is unlikely that these sources of fibrogenic cells provide a major contribution to hepatic extracellular matrix in chronic human liver disease. Cellular sources of proteases that degrade scar and the pathways that regulate them are better understood. Moreover, a more nuanced understanding of distinctive pathogenic features of fibrosis at different stages and from different etiologies means that fibrosis may be customized according to its duration and underlying cause.
Cirrhosis in experimental models and human disease may be reversible.24 Following withdrawal of an injurious stimulus, a dense micronodular cirrhosis can undergo remodeling to a more attenuated, macronodular pattern. However, some septa will persist, likely representing those laid down early in the injury and are therefore the most “mature” (i.e., cross-linked).
Moreover, in experimental models, such mature scars may be the site of neoangiogenesis. Such angiogenesis is already present in chronic inflammatory liver diseases25 concurrent with the fibrogenic process and may also play a role in the pathogenesis of portal hypertension.26 The effectiveness of therapeutic angiogenic inhibitors in not only improving fibrosis, but also in reducing portal pressure, is suggested by data from animal models but has not been established in humans.27 Although there are no data linking septal remodeling to portal pressure changes, recent work correlating increased portal hypertension with smaller nodule size and septal thickening suggests that reversal of these events might lower portal pressure.20
These rodent models and human studies throw into relief the inadequacy of a simple one stage classification, because although the micronodular and remodeled attenuated macronodular cirrhosis are very different, they are both defined by the same original pathologic description: “cirrhosis”. These same experimental models have also facilitated the comparative study of reversible and irreversible septa. Specific septal changes that are associated with irreversibility include: matrix modification with cross-linking, elastin-rich scars, and septal neovascularization. Additionally, the loss of cells that drive matrix turnover from the septa combined with vascular extinction may both limit reversibility. Lastly, of course, the persistence and intensity of the initiating injury will affect the progression of cirrhosis via recurrent cycles of inflammation and repair, regardless of the capacity of the liver to restore a more normal architecture.
Should antifibrotic therapies emerge, the challenges of therapeutically resorbing fibrosis in a cirrhotic liver will be quite different from those of a noncirrhotic liver for several reasons. First, whereas evidence clearly indicates reversibility of fibrosis in precirrhotic disease, the determinants of fibrosis regression in cirrhosis are not sufficiently clear, and the point at which cirrhosis is truly irreversible is not established, either in morphologic or functional terms. Second, there is a heightened sense of urgency in attempting to regress fibrosis in cirrhosis, because continued progression might lead to imminent decompensation, whereas noncirrhotic disease could be decades away from clinical consequences. Thus, the speed of regression in cirrhosis may need to be greater, yet, the cirrhotic liver with its thicker, more cross-linked septa and distorted vasculature may be less amenable to treatment. On the other hand, since fibrosis is part of a chronic wound healing reaction to encapsulate tissue damage, preventing the formation of scar tissue without removing the cause of damage might be detrimental by amplifying the injury. Ideally, therefore, administration of an antifibrotic agent would be most useful when coupled with an effective treatment for the underlying liver disease (e.g., antiviral drugs in patients with HBV or HCV). In contrast, in cirrhotic liver, where the ultimate goal is the reduction of portal pressure, the use of antifibrotic agents coupled with effective treatments to reduce portal pressure and its hemodynamic consequences might be more rational.
Go to:
What Is Cirrhosis?
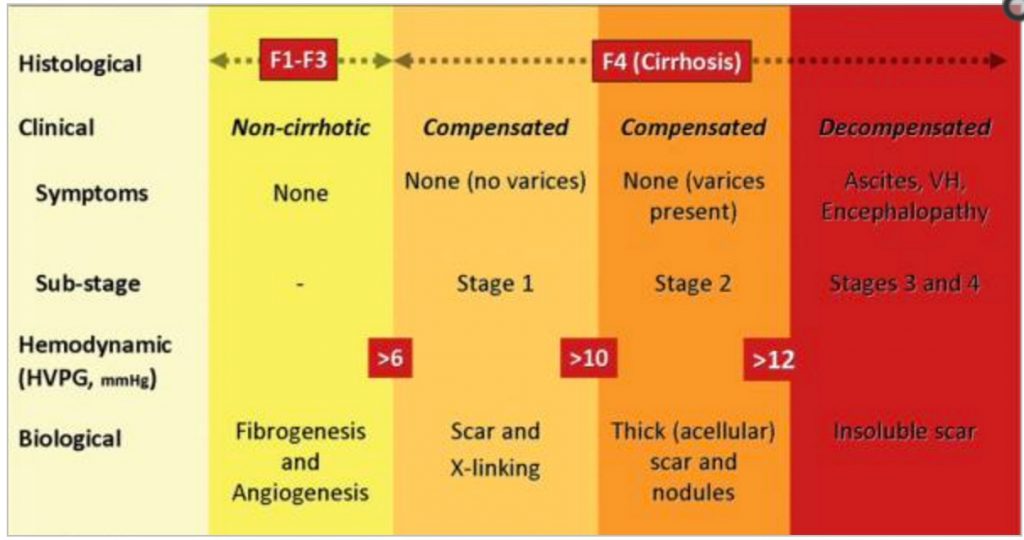
Currently, the diagnosis of cirrhosis in diffuse disease (viral hepatitis, alcohol) relies primarily on histopathological evidence of late-stage fibrosis (e.g., stage 4 fibrosis using the METAVIR system, or stages 5 or 6 in the Ishak scoring system). In this context, and particularly in chronic hepatitis C, sampling errors may lead to underdiagnosis28 or overdiagnosis of cirrhosis.19 Regardless, when using these and related staging systems, “cirrhosis” is a static diagnosis reflecting the end stage of the wound healing process, without adequately signifying the complexity of its pathogenesis, or its functional, hemodynamic and prognostic correlates. Because these collective changes are fundamental to provoking the transition from compensated to decompensated cirrhosis, we need a far more refined pathophysiological classification of compensated cirrhosis based on morphological, functional, and clinical data (Fig. 1).
Fig. 1

Fig. 1
Classification of chronic liver disease based on histological, clinical, hemodynamic, and biological parameters. In the noncirrhotic stage (METAVIR F1–F3), there is no clinical evidence of cirrhosis, the HVPG is below 6 mmHg, and at this stage …
At the least, a revised staging of cirrhosis should start with its main classification of compensated and decompensated cirrhosis. Compensated cirrhosis in turn would comprise two substages: without varices (stage 1) or with varices (stage 2). However, staging of compensated cirrhosis could be further refined as (1) no portal hypertension (HVPG <6 mmHg); (2) portal hypertension that is not clinically significant (HVPG between 6 and 10 mmHg); and (3) clinically significant portal hypertension (HVPG>10 mmHg or presence of collaterals). Substaging of decompensated cirrhosis is not as well-defined but would likely be classified according to both the degree of portal hypertension and the degree of liver/circulatory dysfunction (with recurrent variceal hemorrhage, refractory ascites, and hepatorenal syndrome representing more severe stages) (Fig. 1). It remains possible that additional technologies apart from HVPG will emerge that can further discriminate the pathological and functional state of the liver. Such information could be vital to optimize the timing and nature of antifibrotic therapies, or the need for liver transplantation. Thus far, liver stiffness measurement (LSM) obtained by transient elastography is the most promising noninvasive approach for monitoring fibrosis progression associated with worsening portal hypertension. LSM has an excellent correlation with HVPG values below a threshold of 10–12 mmHg.29,30 Although these findings need to be further substantiated in larger independent studies, they suggest that LSM may be useful in the detection of clinically significant portal hypertension and, thereby, in further subclassifying compensated cirrhosis. On the other hand, LSM may not be accurate in decompensated cirrhosis where, in addition to intrahepatic vascular resistance, there are complex hemodynamic changes.31 Nonetheless, it will be important to evaluate, in longitudinal studies, whether single LSM values or dynamic changes over time are predictive of initial or further decompensation, or the response to pharmacological therapy.32,33
We encourage the practicing community, pathologists, and investigators to move beyond the simple characterization of cirrhosis as a single stage and instead begin thinking of cirrhosis as a series of critical steps that, if left unchecked, culminate in hepatic decompensation. A new framework for classifying cirrhosis will require integration of both current and emerging knowledge about liver structure and function. From one stage, there should emerge many.
Go to:
Abbreviations
HVPG hepatic venous pressure gradient
LSM liver stiffness measurement
Go to:
Footnotes
Potential conflict of interest: Nothing to report.
Go to:
References
1. Pinzani M, Rombouts K, Colagrande S. Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol. 2005;42(Suppl. 1):S22–S36. [PubMed]
2. Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395–414. [PMC free article] [PubMed]
3. Poynard T, Bedossa P, Opolon P, the OBSVIRC MCADg Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. [PubMed]
4. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. [PubMed]
5. Knodell RG, Ishak KG, Black WC, Craig R, Kaplowitz N, Kiernan TW, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. HEPATOLOGY. 1981;1:431–435. [PubMed]
6. Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. [PubMed]
7. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis. A systematic review of 118 studies. J Hepatol. 2006;44:217–231. [PubMed]
8. Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927–934. [PubMed]
9. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. HEPATOLOGY. 2006;43(2 Suppl. 1):S121–S131. [PubMed]
10. Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. [PubMed]
11. Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. [PubMed]
12. Groszmann RJ, Bosch J, Grace N, Conn HO, Garcia-Tsao G, Navasa M, et al. Hemodynamic events in a prospective randomized trial of propranolol vs placebo in the prevention of the first variceal hemorrhage. Gastroenterology. 1990;99:1401–1407. [PubMed]
13. Casado M, Bosch J, Garcia-Pagan JC, Bru C, Banares R, Bandi JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296–1303. [PubMed]
14. Bosch J, Garcia-Pagan JC. Prevention of variceal rebleeding. Lancet. 2003;361:952–954. [PubMed]
15. Moitinho E, Escorsell A, Bandi JC, Salmeron JM, Garcia-Pagan JC, Rodes J, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626–631. [PubMed]
16. Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923–928. [PMC free article] [PubMed]
17. Krogsgaard K, Gluud C, Henriksen JH, Christoffersen P. Correlation between liver morphology and portal pressure in alcoholic liver disease. HEPATOLOGY. 1984;4:699–703. [PubMed]
18. Van Leeuwen DJ, Howe SC, Scheuer PJ, Sherlock S. Portal hypertension in chronic hepatitis: relationship to morphological changes. Gut. 1990;31:339–343. [PMC free article] [PubMed]
19. Blasco A, Forns X, Carrion JA, Garcia-Pagan JC, Gilabert R, Rimola A, et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. HEPATOLOGY. 2006;43:492–499. [PubMed]
20. Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111–117. [PubMed]
21. Sreenivasan P, Inayat I, Jain D, Bari K, Garcia-Tsao G. Histologicalclinical correlation in cirrhosis—Validation of a histological classification of the severity of cirrhosis [Abstract] HEPATOLOGY. 2007;46(Suppl. 1):579A.
22. Viola A, Jain D, Garcia-Tsao G. Quantitative histological assessment in cirrhosis: septal thickness predicts clinical decompensation [Abstract] J Hepatol. 2009;50:S94.
23. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. [PMC free article] [PubMed]
24. Friedman SL, Bansal MB. Reversal of hepatic fibrosis – fact or fantasy? HEPATOLOGY. 2006;43(2 Suppl 1):S82–S88. [PubMed]
25. Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. HEPATOLOGY. 2004;39:1185–1195. [PubMed]
26. Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. [PubMed]
27. Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. HEPATOLOGY. 2009;49:1245–1256. [PubMed]
28. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. [PubMed]
29. Carrion JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791–1798. [PubMed]
30. Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. HEPATOLOGY. 2007;45:1290–1297. [PubMed]
31. Lim JK, Groszmann RJ. Transient elastography for diagnosis of portal hypertension in liver cirrhosis: is there still a role for hepatic venous pressure gradient measurement? HEPATOLOGY. 2007;45:1087–1090. [PubMed]
32. Bosch J. Predictions from a hard liver. J Hepatol. 2006;45:174–177. [PubMed]
33. Vizzutti F, Arena U, Marra F, Pinzani M. Elastography for the non-invasive assessment of liver disease: limitations and future developments. Gut. 2009;58:157–160. [PubMed]