End-Stage Liver Disease in HIV Disease

This article is a wonderful description of cirrhosis and it’s major symptoms. Everyone with cirrhosis should read this and learn from it.
Craig
Liver disease is the most common non–AIDS-related cause of mortality in HIV-infected patients. HIV-infected patients with chronic liver disease progress more rapidly to cirrhosis, and those with hepatitis B virus or hepatitis C virus coinfection progress more rapidly from decompensation to death and are at increased risk of death from end-stage liver disease.
With improvements in health associated with antiretroviral therapy, liver transplantation is increasingly an option in HIV-infected patients with end-stage liver disease. Elements of management of decompensated liver disease, including staging, treatment of variceal hemorrhage and ascites, and considerations in transplantation in the HIV-infected patient are discussed.
This article summarizes a presentation made by Marion G. Peters, MD, at the International AIDS Society–USA continuing medical education program held in Chicago in May 2009. The original presentation is available as a Webcast at www.iasusa.org.
Liver disease is the single greatest cause of non–AIDS-related death in patients with HIV disease, accounting for a greater proportion of deaths than cardiovascular disease or non–AIDS-related cancers (Weber et al, Arch Intern Med, 2006).
Progression to cirrhosis is more rapid in HIV-infected patients with chronic liver disease, and end stage liver disease (ESLD) is now common. A major contributor to ESLD in HIV-infected patients is the high rate of coinfection with hepatitis C virus (HCV) or hepatitis B virus (HBV), estimated at 30% and 10%, respectively. Progression to cirrhosis is accelerated in coinfected patients, and risk of death due to ESLD is markedly increased by coinfection. One recent study showed a dramatic reduction in time from first decompensation to death for patients with HIV and HCV coinfection versus those with HCV infection alone, with 54% versus 74% surviving 1 year, 40% versus 61% surviving 2 years, and 25% versus 44% surviving 5 years, respectively (Pineda et al, Hepatology, 2007).
Another showed that proportions of deaths due to ESLD in HIV-infected persons were 1.2% in those with neither HBV nor HCV coinfection, 22% in those with HBV coinfection, 31% in those with HCV coinfection, and 44% in those with HBV and HCV coinfections (Salmon-Ceron et al, J Hepatol, 2005).
Immune reconstitution with antiretroviral therapy has had a substantial effect in reducing risk of liver-related mortality associated with HCV infection compared with that in patients receiving early antiretroviral therapy or those receiving no treatment before the advent of antiretroviral therapy (Qurishi et al, Lancet, 2003).
Largely as a result of improvement in health and life expectancy with antiretroviral treatment, orthotopic liver transplantation is increasingly considered as an option in HIV-infected patients with liver disease. In addition, successful therapy for HBV and HCV infections can reverse fibrosis and halt progression to cirrhosis and in some cases even reverse cirrhosis and its complications.
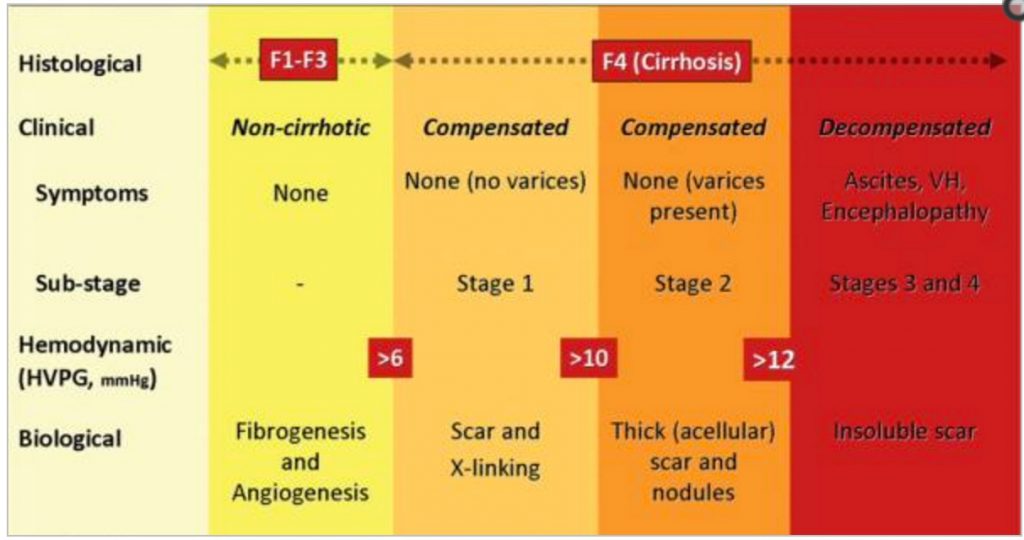
Natural History of End-Stage Liver Disease and Disease Staging Chronic liver disease from any cause (eg, alcohol, HBV or HCV coinfection, nonalcoholic steatohepatitis, or cholestatic or autoimmune causes) results in increasing fibrosis, with a proportion of patients progressing to compensated cirrhosis and a proportion of these progressing to hepatocellular carcinoma (HCC) or decompensated disease with variceal hemorrhage, ascites, encephalopathy, or jaundice.
Patients with decompensation are more likely to die. HCC increases risk of decompensation and risk of death in those with decompensation. Over all, an estimated 5% to 7% of patients with compensated cirrhosis progress to decompensated disease each year.
Currently, the best predictor of decompensation is a hepatic venous pressure gradient (HVPG) of greater than 10 mm Hg; however, measurement of HVPG requires an invasive technique (placing a catheter through the heart and wedging it in the hepatic vein) and thus is not performed routinely outside of several US centers.
The Child-Pugh-Turcotte (CPT) score and Model for End-Stage Liver Disease (MELD) score are widely used tools for assessing disease severity and predicting death in patients with decompensated cirrhosis. The CPT score, developed in the 1970s to assess risk of death after variceal bleeding or portacaval shunting, includes grading of encephalopathy and ascites, albumin level, bilirubin level, and prothrombin time or international normalized ratio (INR; Table 1).
Class A (CPT score, 5−6) indicates well-compensated cirrhotic disease, with progressively worse disease indicated by classes B (score, 7−9) and C (score, >9).
The MELD scoring system, developed subsequently at the Mayo Clinic, avoids the subjectivity involved in grading encephalopathy and ascites by including only bilirubin level, INR, and creatinine level in a mathematic model (Table 2). The initial study of this scale showed that it predicted 3-month mortality after transjugular intrahepatic portosystemic shunting (TIPS), and subsequent studies showed it to be a better predictor of survival of patients on the liver transplant waiting list than was the CPT score.
For approximately the past 10 years, the MELD score has been used to determine order on the liver transplantation waiting list.
Increases in INR and creatinine have a greater effect on the overall score; for example, starting with a normal score of 6, a doubling of bilirubin level would increase the score to 10, a doubling of INR to 20, and a doubling of creatinine level to 27. A further increase in INR would bring the score to 31, which is the current score needed for bloodtype-O patients to receive a transplant in many large centers; non–blood type-O patients are eligible for transplantation at lower scores (in the 20s) at most large centers.
Cirrhosis staging also predicts mortality. Stage 1 is compensated cirrhosis with absence of varices, stage 2 is compensated cirrhosis with varices, stage 3 is decompensated cirrhosis and ascites without variceal hemorrhage, and stage 4 is decompensated cirrhosis and variceal hemorrhage with or without ascites.
Data from a large cohort of untreated patients indicate 1-year mortality rates of 1% for patients in stage 1 disease, 3% in stage 2, 20% in stage 3, and 57% in stage 4.
Varices and Variceal Bleeding
All patients with cirrhosis should be screened for varices by upper endoscopy and treated prophylactically if varices are present. Primary prophylaxis for varices of grade 2 or higher consists of treatment with a nonspecific betablocker (eg, propranolol) to decrease heart rate by 10%. Nonspecific treatment of variceal hemorrhage includes antibiotics (which improve survival) and resuscitation aimed at achieving a hemoglobin level of 9 g/dL (more vigorous resuscitation might serve only to replenish the bleeding varices). Factors associated with increased risk of death within 6 weeks in patients with variceal bleeding in decompensated disease include a MELD score of 18 or greater, the use of 4 or more units of packed red blood cells in the first 24 hours, and active bleeding at endoscopy.
Specific therapies for acute variceal hemorrhage include vasoconstrictors (eg, somatostatin, terlipressin, vapreotide, octreotide) given for 2 days to 5 days in an intensive care unit. Endoscopic varicealband ligation (EVL) to remove varices is now preferred over sclerotherapy, which is associated with a higher risk of esophageal stricture. If these measures fail, TIPS may be performed.
TIPS has largely replaced selective splenorenal shunting, which is now infrequently performed. For prevention of variceal recurrence, the combination of a nonselective betablocker (eg, propranolol) and EVL is superior to EVL alone, with a rebleeding rate of 12% to 14% observed with the combination versus 29% to 38% with EVL alone. The effect of vasoconstrictors in this setting is to reduce splanchnic flow and pressure disproportionately to the reduction in blood pressure, with a goal of reducing the pulse rate by 10% (Table 3).
In contrast, TIPS or another shunting procedure results in increased flow while markedly reducing resistance and portal pressure.
Ascites
The increase in intrahepatic resistance in cirrhosis leads to portal hypertension, splanchnic and systemic vasodilation, a reduction in effective arterial blood volume, activation of neurohumoral systems, and sodium retention and thus to ascites (Figure 1). Interventions in this cascade of events include TIPS to reduce portal hypertension, improvement of effective arterial blood volume with albumin or peritoneovenous shunting, use of spironolactone with or without furosemide to reverse sodium retention, and large volume paracentesis or peritoneovenous shunting to reverse ascites.
When TIPS is used for variceal bleeding, portal pressure drops promptly (with bleeding absent when the pressure falls below 12 mm Hg), but ascites, when present, may not reverse for some 4 weeks to 6 weeks. Thus, although portal hypertension is clearly important in the generation of ascites, simply reducing portal pressure is not sufficient for prompt reversal.
Ascites is staged, by order of increasingly abnormal circulatory state, as diuretic-responsive ascites, refractory ascites, hyponatremia, or hepatorenal syndrome (HRS). Treatment of diuretic-responsive ascites includes sodium intake restriction. Patients should ingest less than 2 g of sodium per day, and intake can be checked by measuring sodium in the urine.
If patients report absence of response to diuretics and have 60 mEq of sodium in the urine, they probably need information regarding limiting the salt content of their food. (Unfortunately, the bottom line in this regard is very close to “if it tastes good, don’t eat it.”)
Treatment also includes spironolactone at a starting dose of 75 mg to 100 mg and furosemide at a starting dose of 20 mg to 40 mg. Spironolactone increases serum potassium and furosemide reduces it, and the effects of the 2 drugs need to be balanced to keep potassium at a normal level. Doses of spironolactone can be increased up to 300 mg to 400 mg if necessary.
With progressive decompensation, ascites becomes refractory. Treatment of refractory ascites may involve large volume paracentesis with 25% albumin (50 mL/L); that is, for every liter of fluid taken off the patient, there must be replacement of 50 mL of 25% albumin, otherwise the patient will become severely catabolic.
As an alternative, TIPS can be performed. Although TIPS is associated with greater transplant-free survival than is paracentesis, it also has a much higher rate of hepatic encephalopathy. The risk of hepatic encephalopathy reflects the fact that the shunting of blood away from the liver to the systemic circulation permits toxins and other substances from the gut (that would ordinarily be metabolized by the liver) to be delivered to the brain.
The goal of TIPS is to reduce portal pressure to below 12 mm Hg, and the procedure is effective in approximately 80% of patients.
There have been a few reports of good outcomes with the combination of albumin with the vasoconstrictors midodrine and octreotide. Experimental treatments include clonidine and vasopressin-2 receptor antagonists. In hyponatremia, treatment consists of fluid restriction and vasopressin-2 receptor antagonists or midodrine. Hypertonic saline should not be used; patients with hyponatremia have normal levels of total body sodium with massive fluid overload, and hypertonic sodium exacerbates the problem.
HRS results from renal vasoconstriction (characterized by decreased cortical flow and increased medullary flow) in response to vasodilation and a marked reduction in effective arterial blood volume. Acute renal failure occurs in 14% to 25% of hospitalized patients with cirrhosis. HRS is the primary form of prerenal failure and accounts for 60% to 80% of cases, with acute tubular necrosis accounting for 20% to 40%.
The rapidly progressive form of HRS (type 1) results in acute renal failure within 2 weeks and is associated with a doubling of serum creatinine level to greater than 2.5 mg/ dL or halving of creatinine clearance to below 20 mL/min. It occurs in patients with refractory ascites, hyponatremia, or both. Prognosis is less than 50% survival at 1 month.
The slower-progressing form of HRS (type 2) is characterized by an increase in serum creatinine level to greater than 1.5 mg/dL (which may be precipitated by excessive diuresis of patients), creatinine clearance of less than 40 mL/min, and urine sodium level of less than 10 mEq, and it is associated with ascites that is unresponsive to treatment with diuretics. Median survival of patients with type 2 HRS is approximately 6 months.
Treatment for HRS consists of liver transplantation. In type 2 HRS associated with extreme splanchnic and systemic vasodilation, treatment with midodrine and octreotide may be successful in reversing vasodilation; albumin is given to increase intravascular volume. These measures rarely have benefit in type 1 HRS. Dialysis is performed in patients with type 1 HRS who are on the transplant waiting list; many of these patients are hypotensive, cannot tolerate intermittent hemodialysis, and are thus treated with continuous veno-venous hemodialysis in an intensive care unit.
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis (SBP) is the most common type of bacterial infection in hospitalized patients, with Escherichia coli the most common pathogen. Clinical suspicion is raised by unexplained encephalopathy, jaundice, and worsening renal failure. In less than 50% of cases, there is fever, abdominal pain or tenderness, and leukocytosis.
Diagnosis is made by tapping the ascites—that is, taking off 20 mL to 30 mL of fluid—and performing a white blood cell (WBC) count and culture. For a count greater than 500 WBCs/µL or a polymorphonuclear cell count greater than 250/µL, antibiotic treatment should be started immediately, prior to receipt of culture results. For culture, samples should be placed in blood culture bottles at the bedside because positive findings are made far more frequently using this approach than by sending the culture to the laboratory. Gram staining is not useful.
Initial treatment is with cephalosporins, with adjustment based on sensitivity testing results. Renal dysfunction is the main cause of death. Intravenous albumin administration can prevent HRS and death in patients with a serum bilirubin level greater than 4 mg/ dL, serum creatinine level greater than 1 g/dL, or blood urea nitrogen level greater than 30 mg/dL.
Recurrence can be prevented by treatment with ciprofloxacin, trimethoprim-sulfamethoxazole, or norfloxacin. Primary prophylaxis with weekly ciprofloxacin is warranted in patients with decompensation and ascites and may be advisable in any patient with a MELD score greater than 9.
Hepatic Encephalopathy
Hepatic encephalopathy is classified as episodic, persistent, or minimal (previously, acute, chronic, and subclinical). It results from a combination of portosystemic shunting and failure to metabolize neurotoxic substances. The nature of these substances remains unclear after decades of research.
Ammonia is not the substance but remains the most important neurotoxic substance that can be measured in the blood. Ammonia levels actually correlate poorly with stage of encephalopathy, although high levels of arterial ammonia do correlate with risk of death in patients with fulminant hepatic failure.
Precipitants of encephalopathy include infection (eg, SBP), gastrointestinal bleeding (ie, increased protein load in the gut), electrolyte imbalance, portal vein thrombosis, worsening liver disease, and shunting.
Treatment is aimed at reducing production of ammonia (and other toxins) from the colon through use of nonabsorbable disaccharides (eg, lactulose, lactitol, lactose) and nonabsorbable antibiotics such as neomycin and rifamixin; data indicate that rifamixin is less absorbable than neomycin.
Protein restriction, once a common treatment, is no longer recommended because it promotes protein degradation, can worsen nutritional status, and can decrease muscle mass when maintained for long periods.
Hepatocellular Carcinoma Monitoring
All patients with cirrhosis should be monitored for HCC. HCC can occur in the absence of severe fibrosis or cirrhosis in HBV disease; thus patients with HBV infection who are older (eg, > 40 years) or have a family history of cancer should also undergo screening. The screening strategy consists of alphafetoprotein testing and ultrasonography every 6 months to 12 months (every 6 months in patients on the transplant waiting list), with quadruple–phase computed tomography for confirmation of abnormalities.
The relative infrequency of ultrasonographic monitoring is based on the relatively low rate of progression from cirrhosis to HCC (approximately 1%−4% per year) and the relatively slow growth rate of HCC tumors (estimated doubling time, 136 days).
Liver Transplantation in HIVInfected Patients
The indication for liver transplantation, in both HIV-infected and noninfected patients, is development of decompensation (ascites, variceal hemorrhage, hepatic encephalopathy), which is associated with a median survival of 1.5 years.
Eligibility for transplantation is based on MELD score and serum sodium level. These criteria likely underestimate the need for transplantation in patients with chronic encephalopathy, hepatic hydrothorax, hepatopulmonary syndrome, or portopulmonary hypertension.
Liver transplantation is now more frequently considered in HIV-infected patients with chronic liver disease because of improvements in health associated with antiretroviral therapy and reflected in reduced mortality, reduced incidence of opportunistic infections, and reduced hospitalization rates from HIV.
Immunosuppressive treatment for transplantation (eg, cyclosporine, mycophenolate mofetil, rapamycin) may also have anti-HIV effects. In addition, better prophylaxis for opportunistic infections is now available. In HIV-infected patients who have undergone liver transplantation, recurrent HBV infection is controlled with combination nucleoside or nucleotide analogue reverse transcriptase inhibitors and HBV immune globulin, such that posttransplantation survival in patients coinfected with HIV and HBVis similar to that in HBV-monoinfected patients.
Recurrent HCV infection, however, is a serious problem, with patients coinfected with HIV and HCV having a higher risk of morbidity and mortality from recurrent HCV infection than have patients with HCV monoinfection. Human papilloma virus (HPV)-associated anal cancer is another major concern. Patients with low-grade cellular abnormalities have progressed to anal cancer during immunosuppression for liver transplantation, and thus patients should be carefully monitored for HPV-associated changes before transplantation.
Similarly, Kaposi sarcoma is a major concern, with transplantation not advised in patients with systemic disease. Patients receiving protease inhibitor–based antiretroviral regimens require major adjustments in dosing of calcineurin inhibitors (eg, cyclosporine, tacrolimus). In this regard, close collaboration between HIV physicians and transplant physicians is necessary to avoid drug interactions that pose the risk of organ rejection or calcineurin toxicity, with a major danger being failure to communicate changes in antiretroviral therapy to the transplant team.
Presented by Dr Peters in May 2009. First draft prepared from transcripts by Matthew Stenger.
Reviewed and edited by Dr Peters in August 2009.
Financial disclosure: Dr Peters has no relevant financial affiliations to disclose.
Source: https://www.iasusa.org/sites/default/files/tam/17-4-124.pdf