New Guideline Addresses Diagnosing, Managing NAFLD
Diagnosing and Managing NAFLD
By: MARY ANN MOON, Family Practice News Digital Network
A new practice guideline for diagnosing and managing nonalcoholic fatty liver disease has been released jointly in the June issues of Gastroenterology, the American Journal of Gastroenterology, and Hepatology.
The guideline represents a collaboration among three societies – the American Gastroenterological Association, the American Association for the Study of Liver Diseases, and the American College of Gastroenterology – and it includes 45 recommendations for physicians and other clinicians. These recommendations “suggest preferred approaches to the diagnostic, therapeutic, and preventive aspects of care.” Summaries of the current world literature upon which these recommendations are based are also included.
The recommendations “are intended to be flexible and adjustable for individual patients,” said Dr. Naga Chalasani, chair of the writing group and director of the division of gastroenterology and hepatology, Indiana University, Indianapolis, and his associates.
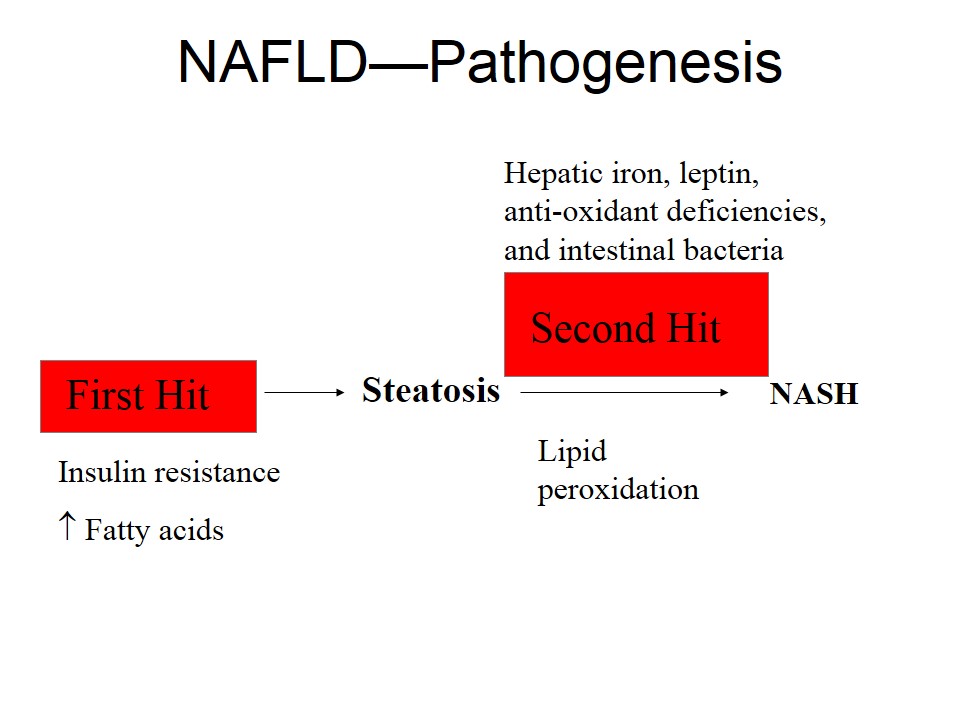
NAFLD is becoming an increasing concern as the obesity epidemic continues. The guideline also includes a discussion of the incidence of NAFLD in the general population and in high-risk groups, as well as a description of the natural history of the disorder, Dr. Chalasani and his colleagues said (Gastroenterol. 2012 May 15 [doi: 10.1053/j.gastro.2012.04.001 ]).
Screening and Diagnosis in Adults
Among the recommendations concerning diagnosis in the guideline are the following points:
• By definition, NAFLD indicates that patients do not have any ongoing or recent intake of significant quantities of alcohol. But until now, the precise definition of “significant” in this context has been uncertain. The guideline states that when assessing patients suspected of having NAFLD, “significant alcohol consumption” should be defined as more than 21 drinks per week in men and more than 14 drinks per week in women.
• Screening for NAFLD is not recommended at this time, either for adults presenting to primary care practices or for high-risk patients attending diabetes or obesity clinics. There are too many uncertainties concerning diagnostic tests and treatment options, and the long-term benefits and cost-effectiveness of screening are not yet known.
• Screening of family members of patients who have NAFLD is not yet recommended for the same reasons, even though there is some evidence suggesting that the disorder is somewhat heritable and can cluster in certain families.
• When evaluating a patient suspected of having NAFLD, it is essential to exclude other possible causes of steatosis as well as to identify possible comorbid liver disease. Hepatitis, medication toxicity, Wilson’s disease, malnutrition, hemochromatosis, and autoimmune liver disease must be considered in the differential.
• Liver biopsy should be considered in patients at risk for steatohepatitis and advanced fibrosis; in those with persistently high serum ferritin and increased iron saturation, especially if they carry genetic mutations in the C282Y gene; and in patients for whom other etiologies or comorbid liver disease cannot be ascertained without a biopsy. Liver biopsy is not recommended in other patients.
Treatment for Adults
Among the guideline’s recommendations concerning treatment are the following:
• It appears that weight loss of at least 3%-5% of total body weight is necessary to reduce steatosis, and that weight loss of 10% is necessary to improve necroinflammation.
• Metformin is not recommended because it has no significant effect on liver histology.
• Pioglitazone can be used in NAFLD patients who have progressed to nonalcoholic steatohepatitis (NASH), but the long-term safety and efficacy of the drug have not been established, and pioglitazone’s effects in patients with diabetes have never been examined.
• Vitamin E should be considered a first-line therapy for NAFLD patients with NASH, but not those who have concomitant diabetes, NASH cirrhosis, or cryptogenic cirrhosis.
• Ursodeoxycholic acid is not recommended for either NAFLD or NASH. Omega-3 fatty acids are not recommended for NAFLD or NASH but may be first-line agents to treat hypertriglyceridemia in patients who have NAFLD.
• Bariatric surgery is not contraindicated unless patients have established cirrhosis, but “the type, safety, and efficacy of foregut bariatric surgery” in NAFLD have not yet been determined.
• Patients with NASH cirrhosis should be screened for gastroesophageal varices and considered for hepatocellular carcinoma screening.
Pediatric Recommendations
The new guideline contains a section devoted to NAFLD in children and adolescents, since the disorder has been reported in children as young as age 2, and NASH-related cirrhosis has been reported in children as young as age 8.
Among the guideline’s recommendations in the pediatric population are the following:
• No formal recommendation regarding screening of overweight and obese children for NAFLD can yet be made because there is too little evidence to support it, “despite a recent expert committee recommendation for biannual screening for liver disease with liver enzyme measurements in this population.”
• Very young or normal-weight children found to have fatty liver should be assessed for monogenic causes of chronic liver disease (such as fatty acid oxidation defects, lysosomal storage diseases, and peroxisomal disorders), as well as the potential causes usually considered among adults.
• High serum titers of autoantibodies, particularly when associated with high aminotransferase and high globulin levels, require a liver biopsy to identify possible autoimmune hepatitis.
• The histopathology of NAFLD in children can differ from that in adults, and can include marked macrovesicular hepatocellular steatosis, portal inflammation, and portal fibrosis in the absence of ballooning.
• Addressing obesity is the first step in treatment, and intensive lifestyle modification is recommended, although no particular diet or exercise program is advocated at this time.
• Metformin is not recommended because it has not been shown to benefit children.
• Vitamin E cannot be recommended for children until confirmatory studies verify that it improves liver histology in NASH.
Dr. Chalasani and his coauthors disclosed relationships with multiple companies; these relationships include research support and paid consulting related to NAFLD.