Primary Biliary Cirrhosis – A MUST read!
 What is PBC?
What is PBC?
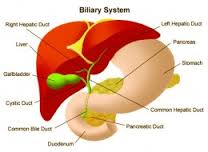
Primary biliary cirrhosis (PBC) is a chronic (long duration) disease characterized by progressive inflammation and destruction of the small bile ducts within the liver. What are the bile ducts and what do they do? Lined with cells named biliary epithelial cells, the bile ducts are tubules that make up a plumbing system for the liver. The bile ducts along with the gallbladder are part of what is called the biliary tract.
The plumbing system begins in the liver with very small caliber ducts that connect to increasingly larger caliber ducts, like a tree in which twigs connect to small branches that connect to larger branches. In fact, this system is often referred to as the biliary tree. The large right and left bile ducts, still within the liver, connect to an even larger common bile duct that runs outside the liver to the small intestine just beyond the stomach. The common bile duct connects by the cystic duct to the gallbladder. The gallbladder is a pear-shaped, expandable, sac-like organ in the biliary system. The branching bile ducts course through special tissue in the liver, called portal tracts, which act like conduits for the ducts. In fact, the branching portal tracts containing the bile ducts also contain the blood vessels that enter and leave the liver.
The bile ducts carry bile, a fluid that is produced by the liver cells (hepatocytes) and modified by the biliary lining (epithelial) cells as it flows through the ducts to the small intestine. Bile contains substances required for digestion and absorption of fat called bile acids, as well as other compounds that are waste products, such as the pigment bilirubin. (Bilirubin is a yellow-orange compound produced by the breakdown of hemoglobin from old red blood cells.) Bile is stored in the gallbladder between meals and discharged into the small intestine during digestion of the meals.
The inflammation in PBC starts in the liver’s portal tracts and involves the small bile ducts in these areas. The destruction of the small bile ducts blocks the normal flow of bile into the gut. The medical term for decreased flow of bile is cholestasis. (Chole means bile and stasis means failure to flow.) Cholestasis is a very important aspect of this disease. As the inflammation continues to destroy more of these bile ducts, it spreads to destroy nearby liver cells (hepatocytes). As the inflammatory destruction of the hepatocytes proceeds, scar tissue (fibrosis) forms and spreads through the areas of destruction.
The combined effects of progressive inflammation, scarring, and toxicity of bile trapped within hepatocytes (liver cells) culminates in cirrhosis. Cirrhosis is defined as the stage of disease when there is both widespread scarring of the liver and clusters (nodules) of hepatocytes reproducing (regenerating) themselves within the scars. Since cirrhosis occurs only in the later stage of PBC, the name primary biliary cirrhosis is actually a misnomer for patients in the earlier stages of the illness. The more technically correct and ponderous term for PBC, chronic non-suppurative destructive cholangitis, however, has never been widely used and is unlikely to replace PBC.
What is the scope of the problem?
PBC is a disease that disproportionately affects women, with 10 women for every man having the disease. It is also a disease of adulthood that, rather curiously, has never been diagnosed in childhood. As a matter of fact, the diagnosis is made most frequently in middle-aged people, between the ages of about 30 and 60 years. PBC is considered to be an uncommon disease, but not rare. Studies indicate that the number of people with PBC at a given time (referred to as the prevalence of disease) ranges from 19 to 251 per million population in various countries. If these figures are adjusted to compensate for the fact that PBC is found only in adults and that 90% of the patients are women, then the calculated prevalence is approximately 25 to 335 per million women and 2.8 to 37 per million men.
The largest and best long-term studies of PBC have been conducted in northern England. Their findings indicate that the number of new cases of PBC over time (referred to as the incidence of disease) has increased steadily from 16 per million population in 1976 to 251 per million in 1994. Unfortunately, no similar studies have been conducted elsewhere to validate or refute the belief that the incidence and prevalence of PBC is rising worldwide.
One comprehensive study conducted in the north of England from 1987 to 1994 was designed specifically to find people with PBC. Using strict criteria for the diagnosis of PBC, they identified a total of 770 patients. Of these, the number of newly diagnosed people with PBC during just these 7 years was 468. Thus, clinical investigators interested in PBC had conducted extensive epidemiological (cause and distribution) studies of PBC over almost 20 years in this same geographic area. Such a concentrated focus of effort strongly supports the view that the apparent increase in the number of people with PBC is indeed a true increase.
What is the cause of PBC?
The cause of PBC remains unclear. Current information suggests the cause may involve autoimmunity, infection, or genetic (hereditary) predisposition, acting either alone or in some combination. A complete understanding of the cause of PBC will require two types of information. One, referred to as the etiology, is identification of the initiating (triggering) events. The other, referred to as the pathogenesis, is a discovery of the ways (mechanisms) by which the triggering events lead to the inflammatory destruction of bile ducts and hepatocytes. Unfortunately, neither the etiology nor the pathogenesis of PBC has yet been defined.
PBC is presumed by most experts to be an autoimmune disease, which is an illness that occurs when the body’s tissues are attacked by its own immune (defense) system. (Auto means self.) Childhood diabetes is one example of an autoimmune disease in which some type of transient infection (one that later goes away) triggers an immune reaction in a susceptible (genetically predisposed) person. This particular immune reaction in diabetes selectively destroys the cells in the pancreas that produce insulin.
Despite strong evidence to support the concept that PBC likewise is an autoimmune disease, some features of PBC are uncharacteristic of autoimmunity. For example, all other autoimmune diseases occur in both children and adults, while, as already mentioned, PBC has never been diagnosed in childhood. PBC and other autoimmune diseases, however, are associated with antibodies (small proteins found in the blood and bodily secretions) that react with the body’s own proteins, which are referred to as autoantigens.
Specific types of white blood cells called B-lymphocytes make antibodies. Antibodies recognize specific protein targets called antigens (substances that are capable of causing the production of antibodies.) To facilitate our discussion of autoimmunity, let us first look at what happens in the more common type of immunity. It takes new or foreign antigens to produce this usual type of immunity. Vaccines, infectious organisms (like viruses or bacteria), or surgically transplanted tissues contain such foreign antigens. So, for instance, when a person is first vaccinated to prevent tetanus, that person is newly exposed to tetanus proteins, which are foreign antigens. What happens then?
First, specialized cells within tissues of the body take up and digest the tetanus proteins. Then the protein fragments are attached to special molecules called HLA molecules that are produced by the HLA complex. (HLA is an abbreviation for Human Leukocyte Antigen). The HLA complex is a group of inherited genes located on chromosome 6. The HLA molecules control a person’s immune response. Next, the protein (antigen) fragments bound to the HLA molecules set into action (activate or stimulate) specialized white blood cells called T-lymphocytes. The T-lymphocytes then begin to multiply (reproduce) and secrete chemical signals into their environment.
Another type of white blood cell, called B-lymphocytes, also enters the picture. B-lymphocytes have molecules on their surface, called immunoglobulins (Ig) that can bind directly to undigested tetanus antigens. An essential part of the body’s immune system, immunoglobulins are antibodies that attach to foreign substances, such as bacteria, and assist in destroying them. This binding activates the B-lymphocytes, that is, gets them ready for action. Meanwhile, the above-mentioned secreted chemicals of the activated T-lymphocytes provide a helper signal for the B-lymphocytes. This signal tells the B-lymphocytes to begin secreting the immunoglobulins (specific antibodies) that precisely recognize the stimulating tetanus antigen.
The bottom line here is that antibodies that specifically bind and inactivate tetanus proteins prevent an immunized person from developing tetanus. What is more, both the T- and B-lymphocytes reside in the body as memory cells. This means that they can remember to generate increased amounts of antibodies against tetanus antigens whenever a person has a booster shot of the vaccine. So, that’s what happens in the common type of immunity.
By contrast, in autoimmunity, autoantibodies, produced by B-lymphocytes react against self or auto antigens rather than against foreign antigens. In this reaction, the activated B-lymphocytes still require help from chemicals secreted by activated T-lymphocytes. Although the human immune system is capable of recognizing a nearly infinite number of antigens, normally it does not recognize or respond to autoantigens. The expected absence of immune responses against self is called tolerance.
Thus, in all autoimmune diseases, including PBC, tolerance (absence of an immune response) becomes defective (is lost) for autoantigens recognized by both T- and B-lymphocytes. In other words, an immune response to autoantigens does occur. What’s more, in autoimmune diseases, B-lymphocytes initially produce autoantibodies that recognize a single autoantigen. With time, however, B-lymphocytes produce new autoantibodies that recognize additional autoantigens that are distinct from the initial autoantigen. PBC, however, is the only allegedly autoimmune disease in which this sequence does not occur. In other words, in PBC, the autoantibodies recognize only the initial autoantigen.
What are antimitochondrial antibodies (AMA)?
Between 95 and 98% of patients with PBC have autoantibodies in their blood that react with the inner lining of mitochondria. These autoantibodies are called antimitochondrial antibodies (AMA). Mitochondria are the energy factories present inside all of our cells, not just the cells of the liver or bile ducts. The mitochondria use the oxygen carried in the blood from the lungs as a fuel to generate energy. AMA bind to protein antigens that are contained in multienzyme complexes (packages of enzymes) within the inner lining of the mitochondria. The multienzyme complexes produce key chemical reactions necessary for life. These complexes are referred to as multienzyme because they are made up of multiple enzyme units.
AMA specifically react against a component of this multienzyme complex called E2. In PBC, AMA preferentially react with the E2 component of one of the multienzymes that is called the pyruvate dehydrogenase complex (PDC). Accordingly, the antigen is designated as PDC-E2. The practical importance of all of this is that the PDC-E2 antigen is now used, as will be discussed below, in a diagnostic test for detection of AMA. The PDC-E2 antigen is also referred to as M2, a term introduced to designate it as the second mitochondrial antigen discovered by researchers interested in PBC.
Do the AMA react with the bile ducts?
In as much as the bile ducts are the main targets of destruction in PBC, the question was asked whether the AMA reacts with the epithelial cell lining of the bile ducts. So, investigators prepared antibodies to PDC-E2. As expected, they found that these antibodies bound to the mitochondria within cells. But, sure enough, recent information suggests that these AMA autoantibodies also bind to PDC-E2 that lies outside the mitochondria yet within the epithelial cells lining the bile ducts. Indeed, these cells are the main targets of destruction in PBC.
This accumulation of PDC-E2 within the biliary epithelial cells is observed exclusively in the livers of patients with PBC, and not in normal livers or in livers from patients with any other types of liver disease. Interestingly, it was also observed in the livers of those two to five percent of PBC patients who do not have AMA in their blood (AMA-negative PBC). Furthermore, intense binding of these antibodies to biliary epithelial cells was also found to be the earliest indication of recurrence of PBC in a transplanted liver. (PBC is sometimes treated by liver transplantation, which will be discussed later.)
These observations led to a speculation that the antibodies were actually reacting with an antigen from an infectious agent. The idea was that the infectious agent was present in the biliary epithelial cells of patients with PBC and that the agent could also infect the biliary cells of a transplanted liver. (See the section below on the role of infection).
What causes destruction of the bile ducts in PBC?
AMA are tremendously important as a diagnostic marker in patients with PBC. Despite that, no evidence exists that the AMA itself causes the destruction of the biliary epithelial cells lining the small bile ducts. Neither the presence nor the amount (titer) of AMA in the blood appears to be related to the inflammatory destruction of the bile ducts. Indeed, immunization of animals with PDC-E2 antigen results in production of AMA without any liver or bile duct damage (pathology).
What, then, causes the destruction of the bile ducts in PBC? Inspection of liver biopsies from patients with PBC indicates that T-lymphocytes surround and invade the small bile ducts. Thus, T-lymphocytes appear to be responsible for the death of the biliary epithelial cells lining the ducts and the destruction of the bile ducts. T-lymphocytes capable of directly killing target-cells (for example, biliary epithelial cells) are called cytotoxic T-lymphocytes, meaning that these T-cells are toxic to the target cells. And, in fact, cytotoxic T-lymphocytes have been observed in liver biopsies to invade the bile ducts and to be present in areas where biliary epithelial cells are dying.
Other T-lymphocytes that surround the bile ducts are known to produce chemicals that can also cause biliary epithelial cells to die. Some of these chemicals actually stimulate the biliary epithelial cells themselves to secrete small proteins that attract more T-lymphocytes. Paradoxically, then, this response by the biliary epithelial cells might result in even greater injury to the bile ducts, in sort of a vicious cycle.
Recent studies of T-lymphocytes isolated from the inflamed livers of patients with PBC have shown that these T-lymphocytes can, in fact, kill biliary epithelial cells. Moreover, many of the T-lymphocytes recognized the digested fragments of PDC-E2. These observations suggest the possibility (hypothesis) that the T-lymphocytes might attack the biliary epithelial cells because these cells display PDC-E2 antigens in their HLA (Human Lymphocyte Antigen) molecules to which the T lymphocytes react. No direct evidence, however, supports this hypothesis. The fact is that the actual antigens on biliary epithelial cells that are recognized by invading, destructive T-lymphocytes remain to be determined. However, the biliary epithelial cells do contain molecules, such as intercellular adhesion molecule-1, that are required for activated T lymphocytes to adhere to the cells that they kill.
What is the role of infection?
The possibility that PBC is caused by an infection with a virus, bacterium, or fungus has generated a number of studies. To date, none has shown conclusively that PBC is an infectious disease or even that it is triggered by a self-limited (nonpersistent) infection. Clearly, PBC is not associated with infection by any of the known hepatitis viruses. Furthermore, none of the new viruses that may cause liver diseases have been found preferentially or exclusively in patients with PBC.
Investigators are currently pursuing leads suggesting that the biliary epithelial cells of patients with PBC may contain an infectious virus that belongs to the class of viruses called retroviruses. (The human immunodeficiency virus, HIV, is an example of a retrovirus.) These studies have identified genetic fragments of a retrovirus in the biliary epithelial cells of patients with PBC. Nevertheless, further research is required to answer the important question of whether PBC is caused by a retroviral infection.
The possibility that PBC is caused by infection with bacteria has intrigued clinical investigators for decades. You see, the mitochondria in the cells of mammals were derived, during evolution, from bacteria. Thus, many bacteria contain antigens that react with the AMA found in patients with PBC. Some of these bacteria have been cultured from the urine of patients with PBC who have recurrent urinary tract infections. Interestingly enough, as discussed later, recurrent urinary tract infection has been recognized as a risk factor for developing PBC.
This association between urinary tract infection and PBC led to the speculation that a bacterial infection might trigger an immune response that developed into an autoimmune reaction. Although this speculation is plausible, there is currently no direct evidence that this sequence of events occurs in PBC. As a matter of fact, molecular techniques now exist to screen livers for the presence of any type of bacteria. So far, these kinds of studies have found no evidence of a chronic bacterial infection in PBC.
Another intriguing possibility is that an infection with a virus, bacterium, fungus or parasite might introduce foreign proteins that mimic the protein antigens of mitochondria. An immune response against these foreign proteins could develop antibodies and T lymphocytes that react with the mimicked self-proteins, thereby resulting in autoimmunity. In other words, the body’s immune system responds to the foreign proteins but it reacts against its own mitochondrial proteins. This phenomenon is called molecular mimicry.
One of the best examples of molecular mimicry is found in rheumatic fever. This condition is an autoimmune reaction involving the skin, joints, and heart muscle, that is caused by an immune response to a streptococcal bacterial infection. Now, rheumatic fever is usually diagnosed within a few weeks of having strep throat. Physicians, therefore, recognized the relationship between the two events (streptococcal infection and rheumatic fever) before molecular mimicry was understood. PBC, however, is usually a more subtle condition that might not be diagnosed for many years. Therefore, if a transient infection were to trigger molecular mimicry in PBC, causing an autoimmune reaction, the relationship between the infection and the autoimmune disease might be easily missed.
What is the role of genetics?
PBC is not transmitted by heredity from parents with the disease to their children. Thus, PBC is not a classical hereditary (genetic) disease, as is diabetes, for example. Clearly, however, the genes of our immune system control human responses to infections with bacteria and viruses. The genes of the immune system also control the risk of developing autoimmune diseases. Studies have shown that there are some weak associations between PBC and certain specific inherited genes of the immune system. The fact that many people without PBC also have these identical immune genes indicates that the genes themselves do not determine if a patient develops the disease.
Accordingly, it appears likely that some immune genes create susceptibility for PBC, but the disease does not occur without additional events. Besides that, certain other immune genes may control progression of the disease. These genes are more common in patients with advanced PBC than in patients with the earlier stages of PBC. Indeed, recently, additional genes involved in immune signaling were found to be markers of both susceptibility and disease progression. Studies currently being conducted on patients whose close relatives also have PBC may clarify exactly which genes are associated with susceptibility and progression of PBC.
What are the symptoms and physical findings in PBC?
The symptoms and physical signs (findings) in patients with PBC can be divided into those manifestations due to:
PBC itself
Complications of cirrhosis in PBC
Diseases often associated with PBC
Table 2 lists the multiple signs and symptoms (manifestations) of primary biliary cirrhosis, its associated diseases, and the complications of the cirrhosis.
Primary Biliary Cirrhosis Associated Diseases Complications of Cirrhosis
Fatigue
Thyroid dysfunction
Edema and ascites
Itching
Sicca syndrome
Bleeding from varices
Metabolic bone disease
Raynaud’s phenomenon
Hepatic Encephalopathy
Xanthomas
Scleroderma
Hypersplenism
Fat & vitamin malabsorption
Rheumatoid arthritis
Hepatocellular carcinoma
Jaundice
Celiac sprue
Hyperpigmentation
Inflammatory bowel disease
Urinary tract infections
Medical Author: John M. Vierling, MD, FACP
Medical Editor: Leslie J. Schoenfield, MD, PhD
1 Comment
Leave a Comment
You must be logged in to post a comment.
Most comprehensive report I have read on PBC. Thank you. And the great news that it is now called Primary Biliary Cholangitis means that we have lost the stigma of being heavy alcoholic drinkers!!