Liver Cirrhosis
Liver Cirrhosis
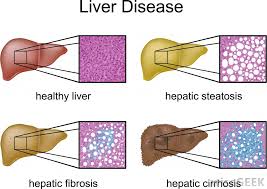
Cirrhosis is defined as the histological development of regenerative nodules surrounded by fibrous bands in response to chronic liver injury, that leads to portal hypertension and end stage liver disease. Recent advances in the understanding of the natural history and pathophysiology of cirrhosis, and in treatment of its complications, resulting in improved management, quality of life and life expectancy of cirrhotic patients.
At present, liver transplantation remains the only curative option for a selected group of patients, but pharmacological therapies that can halt progression to decompensated cirrhosis or even reverse cirrhosis are currently being developed. This concise overview focuses on diagnosis, complications and management of cirrhosis, and novel clinical and scientific developments.
Pathogenesis and pathophysiology of cirrhosis
Fibrosis describes encapsulation or replacement of injured tissue by a collagenous scar. Liver fibrosis results from the perpetuation of the normal wound healing response resulting in an abnormal continuation of fibrogenesis (connective tissue production and deposition). Fibrosis progresses at variable rates depending on the cause of liver disease, environmental and host factors.
Cirrhosis is an advanced stage of liver fibrosis that is accompanied by distortion of the hepatic vasculature. It leads to shunting of the portal and arterial blood supply directly into the hepatic outflow (central veins), compromising exchange between hepatic sinusoids and the adjacent liver parenchyma, i.e., hepatocytes. The hepatic sinusoids are lined by fenestrated endothelia which rest on a sheet of permeable connective tissue (the space of Disse) which contains hepatic stellate cells (HSC) and some mononuclear cells. The other side of the space of Disse is lined by hepatocytes which execute most of the known liver functions.
In cirrhosis the space of Disse is filled with scar tissue and endothelial fenestrations are lost, a process termed sinusoidal capillarization.
Histologically, cirrhosis is characterized by vascularized fibrotic septa that link portal tracts with each other and with central veins, leading to hepatocyte islands that are surrounded by fibrotic septa and which are devoid of a central vein. The major clinical consequences of cirrhosis are impaired hepatocyte (liver) function, an increased intrahepatic resistance (portal hypertension) and the development of hepatocellular carcinoma (HCC). The general circulatory abnormalities in cirrhosis (splanchnic vasodilation, vasoconstriction and hypoperfusion of kidneys, water and salt retention, increased cardiac output) are intimately linked to the hepatic vascular alterations and the resulting portal hypertension. Cirrhosis and its associated vascular distortion are traditionally considered to be irreversible but recent data suggest that cirrhosis regression or even reversal is possible.
Epidemiology
The exact prevalence of cirrhosis worldwide is unknown. Cirrhosis prevalence was estimated at 0.15% or 400,000 in the USA, where it accounted for more than 25,000 deaths and 373,000 hospital discharges in 1998. This may be an underestimation as we recognize the high prevalence of undiagnosed cirrhosis in both NASH and hepatitis C. Similar numbers have been reported from Europe, and numbers are even higher in most Asian and African countries where chronic viral hepatitis B or C are frequent. Since compensated cirrhosis often goes undetected for prolonged periods of time, a reasonable estimate is that up to 1% of populations may have histological cirrhosis.
Etiology of cirrhosis
The etiology of cirrhosis can usually be identified by the patient’s history combined with serologic and histologic evaluation. Alcoholic liver disease and hepatitis C are the most common causes in the Western world, while hepatitis B prevails in most parts of Asia and sub-Saharan Africa. After the identification of the hepatitis C virus in 1989 and of nonalcoholic steatohepatitis (NASH) in obese and diabetic subjects, the diagnosis of cirrhosis without an apparent cause (cryptogenic cirrhosis) is rarely made.
It is important to know the etiology of cirrhosis, since it can predict complications and direct treatment decisions. It also allows the discussion of preventive measures, e.g., with family members of patients with alcoholic cirrhosis or chronic viral hepatitis, and consideration of (genetic) testing and preventive advice for relatives of patients with genetic diseases, such as hemochromatosis or Wilson’s disease.
Frequently multiple etiological factors contribute to the development of cirrhosis, as exemplified in epidemiological studies that identified regular (moderate) alcohol consumption, age above 50 years, and male gender as risk factors in chronic hepatitis C, or older age obesity, insulin resistance/type 2 diabetes, hypertension and hyperlipidemia (all features of the metabolic syndrome) in NASH.
Clinical presentation
Cirrhosis is frequently indolent, asymptomatic and unsuspected until complications of liver disease present. A sizable proportion of these patients never come to clinical attention, and previously undiagnosed cirrhosis is still frequently found at autopsy (14). The diagnosis of asymptomatic cirrhosis is usually made when incidental screening tests such as liver transaminases or radiologic findings suggest liver disease and patients undergo further evaluation and liver biopsy.
The recognition that 20% of HCV patients and perhaps as many as 10% of patients with NASH may progress to cirrhosis has led to the frequent use of biopsy in these high risk groups prior to development of clinical signs of cirrhosis. However, initial clinical presentation of patients with decompensated cirrhosis is still common and is characterized by the presence of dramatic and life-threatening complications, such as variceal hemorrhage, ascites, spontaneous bacterial peritonitis, or hepatic encephalopathy.
Imaging of cirrhosis
Ultrasonography, computerized tomography (CT) and magnetic resonance imaging (MRI) are not sensitive to detect cirrhosis, and final diagnosis still relies on histology. However, their specificity is high when an obvious cause is present and imaging reveals an inhomogeous hepatic texture or surface, rarefied hepatic central vein, an enlarged caudate lobe, splenomegaly or collateral veins.
However, other etiologies such as portal vein thrombosis, parasitic diseases or hematological malignancies need to be excluded, and normal radiographic findings do not exclude compensated cirrhosis. The major role of radiography is for the detection and quantitation of complications of cirrhosis, i.e., ascites, HCC, and hepatic or portal vein thrombosis.
Ultrasonography provides important information on hepatic architecture, is cheap and widely available. Nodularity and increased echogenicity of the liver are often found in cirrhosis but are also present in steatosis. There is typically atrophy of the right lobe and hypertrophy of the left and especially caudate lobes. However, the width of the caudate relative to the right lobe is a poor predictor of cirrhosis.
Ultrasonography and Doppler ultrasonography of portal and central vein diameters and velocities are useful screening tests for portal hypertension and vessel patency. Contrast ultrasonography examines the appearance of echogenic microbubbles in the hepatic vein.
Their appearance after antecubital injection is correlated inversely with fibrosis. Ultrasonography is the first imaging modality for suspected HCC, but its sensitivity and specificity to detect HCC is below that of CT or MRI , and nodular lesions should be confirmed by helical CT and/or MRI. A high degree of suspicion, e.g., in patients with an alfa-fetoprotein above 200 μg/L, or pretransplant evaluation requires these more rigorous techniques even in the absence of ultrasonographic lesions. Contrast ultrasonography, harmonic imaging and power Doppler improve detection of HCC via sensitive visualization of abnormal vessels but are not yet generally available.
Conventional CT and MRI are not useful to define the severity of cirrhosis, while helical CT and MRI with contrast are the modalities of choice when HCC or vascular lesions are suspected. In a comparison MRI was superior to helical CT for detection of small HCC of 1-2cm size (39). MRI has also been shown to be effective in determining hepatic iron and fat content in hemochromatosis and liver steatosis, respectively.
Elasticity measurement (Fibroscan) is a promising technique based on the velocity of an elastic wave via an intercostally placed transmitter. Shear wave velocity is determined by pulse ultrasound and correlates with liver stiffness, i.e., fibrosis. The examination is limited by morbid obesity, ascites and small intercostal spaces. In a study of 327 patients with hepatitis C, histological cirrhosis was differentiated from milder stages of fibrosis with a receiver-operating characteristics (ROC) curve of 0.97 which is considered an almost ideal test. Elasticity scans have the ability to sample 1/500 of the liver and represent a useful, non-invasive test for diagnosing or excluding cirrhosis.
Liver biopsy
Biopsy is considered the gold standard for diagnosis of cirrhosis, and sequential histological grading of inflammation and staging of fibrosis can assess risk of progression. However, biopsy is prone to considerable sampling variability in all liver diseases (43-46). Thus when staging fibrosis in hepatitis C patients using the METAVIR system which is simple and uses only 4 stages (stage 4 being cirrhosis), one third of scores differed by at least one stage when a biopsy from the left liver lobe was compared to that from the right lobe, and with similar results for grading of inflammation.
In hepatitis C, correct staging was only achieved for 65% and 75% of cases when biopsies were 15 mm and 25 mm in length, respectively, while in clinical practice only 16% of biopsies reach 25mm in length. Despite these shortcomings, biopsy is still required to confirm cirrhosis in patients with compensated liver function and to suggest its cause. Biopsy confirmation of cirrhosis is not necessary when clear signs of cirrhosis, such as ascites, coagulopathy, and a shrunken nodular appearing liver are present.
A liver biopsy is obtained by either a (radiographically-guided) percutaneous, a transjugular or laparoscopical route. A greater risk of bleeding following a biopsy has been observed with larger-diameter needles. In suspected cirrhosis a cutting is preferred over a suction needle, in order to prevent tissue fragmentation. 2 to 3 percent of patients require hospital admission for management of complications; pain or hypotension are the predominant causes. 60% of complications occur within two, and 96% within 24 hours after biopsy.
Mortality, mainly due to severe bleeding is 1 in 10,000 to 12,000, and likely higher in cirrhosis. Blood products should be replaced when platelets are below 70,000/μL or prothrombin time is prolonged by more than four seconds, and/or a transjugular or laparoscopic approach chosen. Aspirin and other anti-platelet agents should be stopped at least a week before biopsy.
Natural History and Prognosis
The natural history of cirrhosis is dependent on both the etiology and treatment of the underlying cause. Annual rates of decompensation are 4% for HCV, 10% for HBV and the incidence of HCC is between 2 – 7% per year. Decompensation in alcoholic cirrhosis with continued alcohol use is even more rapid and often associated with alcoholic hepatitis on a background of cirrhosis. Once decompensation has occurred, mortality without transplant is as high as 85% over 5 years.
Numerous studies have attempted to develop a classification system that can both characterize the degree of liver injury and predict the prognosis of patients with cirrhosis based on clinical and laboratory parameters. Due to its low level of complexity and its fairly good predictive value, the Child-Pugh-Turcotte (CPT) classification is widely used. One-year survival rates for patients with CPT A, B, and C cirrhosis are 100, 80, and 45 percent, respectively.
CPT class predicts the development of complications, such as variceal hemorrhage and the response of patients to surgical interventions (50). More recently with the pressure in the allocation of scarce liver donors for transplantation, the Model for End Stage Liver Disease (MELD) has been developed to more precisely evaluate short term mortality.
MELD best predicts 3 month survival of cirrhotics, irrespective of etiology. It is based on creatinine, bilirubin and INR, but lacks features of portal hypertension, such as ascites. It gives priority to patients who are most likely to die without a liver transplant, such as those with hepatorenal failure. In the USA the replacement of the former individualized system of organ allocation, which was heavily based on waiting time, by MELD reduced mortality on the waiting list without change in post-transplant outcome.
The system is currently considered for further refinement, such as giving extra points to patients with HCC and hyponatremia <130mEq/mL. CPT and MELD scores can vary greatly when single parameters are modified by medical treatment, such as substitution of albumin, removal of ascites or diuretic treatment. Here, an increasing MELD score over time is a better predictor of cirrhosis severity and progression.
Treatment and Reversibility of cirrhosis
Causal
Elimination of the trigger(s) that lead to cirrhosis is likely to retard progression to a higher CPT class and to reduce the incidence of HCC. There is evidence that causal treatment may even reverse cirrhosis, although in some of the reports sampling variability cannot be excluded.
Patients with alcoholic cirrhosis must abstain, since continued alcohol consumption drives hepatitis which favours hepatic fibrogenesis and decompensation. Liver function often worsens in the first 2-3 weeks of withdrawal, since alcohol has an immunosuppressive effect.
Patients with compensated replicating HCV-cirrhosis benefit from interferon-based antiviral treatment. Viral eradication and a consequently lowered risk of hepatic decompensation and hepatocellular carcinoma can be achieved in up to 40 and 70% of patients with genotypes 1 and 2 or 3, respectively (58). In a recent meta-analysis 75 out of 153 biopsy-proven cirrhotics showed reversal of cirrhosis on biopsy after successful treatment (59), but results need conformation in view of biopsy sampling variability. How far maintenance interferon for 3-4 years can prevent hepatic decompensation or hepatocellular carcinoma in subjects with stage 3 or 4 fibrosis who did not respond to interferon-ribavirin therapy is currently evaluated in large prospective trials (HALT-C, EPIC-3 and COPILOT).
Longterm treatment with oral nucleoside and nucleotide inhibitors of HBV polymerase may not only retard or reverse cirrhosis but were also shown to prevent complications of end stage liver disease. In a 3 year study of lamivudine for HBV, follow up liver biopsies suggested reversal of cirrhosis in 8/11 patients (73%) and in 436/651 patients with HBV-cirrhosis treated with lamivudine for a mean of 32 months a >50% reduction of hard clinical endpoints, as defined by hepatic decompensation, hepatocellular carcinoma, spontaneous bacterial peritonitis, bleeding gastroesophageal varices, or death related to liver disease was attained.
In replicating HBV-cirrhosis (>105 copies/mL) lamivudine treatment frequently resulted in clinical improvement, even after decompensation. The high rate of lamivudine resistance which reaches 56% and 70% after 3 and 4 years of treatment, respectively, is now of lesser concern, since equally well tolerable alternatives like adefovir, entecavir or telbivudine, or their combinations are available which display lower rates of viral resistance and a different mutational profile. In one large study, adefovir treatment was successfully used in patients with lamivudine resistance pre-transplant, leading to suppression of HBV viral replication to undetectable levels in 76% of patients with either a stabilization or improvement in CTP score and a 90% survival.
The data on reversibility and stabilization of other causes of cirrhosis is less well defined. Cohort studies showed that some cirrhotic patients with autoimmune hepatitis showed regression after long-term treatment with corticosteroids, and venesection of patients with hereditary hemochromatosis could decrease the development of complications of portal hypertension
Complications of Cirrhosis
A detailed review of the complications of cirrhosis is beyond the scope of this article. Major advances have been made in recent years to both prevent and treat the common complications of cirrhosis such as variceal bleeding, ascites, spontaneous bacterial peritonitis and encephalopathy (72-78, Table 5). It is important to note that bacterial infections are frequent, especially in decompensated cirrhotics, exacerbating hepatic dysfunction, encephalopathy and portal hypertension and underlining the need for vigilance and rigorous antibiotic treatment in cirrhosis. Enhanced bacterial translocation from the intestine, a compromised immune function and an excessive proinflammatory cytokine release have been implicated in the pathogenesis of the cirrhosis-associated systemic inflammatory syndrome (79). An example is the failure to control esophageal variceal bleeding with associated bacterial infection (80).
Complications of cirrhosis, their prevention and treatment
An important realization for the clinician is that once complications have developed, suitable patients should be referred to a Liver Center that specializes in both the care of patients with end stage liver disease and liver transplantation. Special attention has also to be paid to the circulatory and cardiac abnormalities in cirrhosis that can preclude transplant eligibility. The hepatopulmonary syndrome which occurs in 15-20% of cirrhotics is due to overproduction of NO and overexpression of the endothelin B receptor with consequent pulmonary arteriolar vasodilation and hypoxemia (81,82). It is largely reversible after transplantation.
Portopulmonary hypertension is rare, but its prevalence rises to 16-20% of patients with refractory ascites. It is likely caused by an excess of pulmonary arteriolar vasoconstrictors and profibrogenic factors like TGFβ1 (83). The condition is considered irreversible and a pulmonary artery pressure >40 mmHg precludes liver transplantation (84). Cirrhotic cardiomyopathy is characterized by a blunted stress response of the heart, combined with hypertrophy (85). Severe forms increase postoperative mortality and preclude transplantation.
Hepatocellular Carcinoma
HCC is one of the commonest solid organ tumors worldwide and cirrhosis is the major risk factor for progression to HCC (86-88). Other risk factors are listed in Table 6. The pathogenic appears to be the development of regenerative nodules with small cell dysplasia and then invasive HCC. The mortality rate of HCC associated with cirrhosis is rising in most developed countries, whereas mortality from non-HCC complications of cirrhosis is decreasing (89). Cirrhosis due to HCV is associated with the highest HCC incidence in Japan compared to the West, followed by hereditary hemochromatosis (5-year cumulative incidence 17-30%).
In cirrhosis due to HBV, the major cause for HCC-related deaths in the world, the 5-year cumulative incidence of HCC is 15% in high endemic areas and 10% in the West. 5-year HCC incidence is lower in alcoholic cirrhotics, or in patients with biliary cirrhosis (8% and 4%, respectively). HCC is increasing in the USA, where its incidence had increased from 1.8 per 100,000 to 2.5 per 100,000 over one decade, mainly attributable to HCV infection (90).
Risk factors for hepatocellular carcinoma
Screening for HCC is one of the most important tasks in following patients with cirrhosis. Current AASLD and EASL guidelines recommend at least one annual screening for HCC in patients with cirrhosis using imaging with ultrasound, triphasic CT scan or gadolinium enhanced MRI (86-88). Serum alfa-fetoprotein,which was an integral component of prior screening algorithms, is no longer recommended due to its poor sensitivity and specificity. Once HCC is detected, multiple treatment modalities are available that depend on tumor size, tumor number and local expertise. In the non-cirrhotic patient, surgical resection is an option and can be curative. However, most patients with cirrhosis will not tolerate liver resection or have microscopic satellite lesions, and the best option for cure is with liver transplantation.
The Milan criteria have suggested that the mortality and recurrence rate of HCC is acceptable if liver transplant is performed for either a solitary tumor <5cm in diameter or no more than 3 tumors with the largest being <3cm in diameter. Alternative treatments for HCC patients who do not meet the criteria for surgical resection or transplant are radiofrequency ablation, chemoembolization, alcohol ablation and cyberknife radiotherapy (86-88). Selection of these modalities depends on local expertise, and randomized trials suggesting that they improve long term survival are scarce.
Liver transplantation
The ultimate therapy for cirrhosis and end stage liver disease is liver transplantation. Indications and contraindications for liver transplant are given in Table 5. The most recent survival data from the United Network of Organ Sharing (UNOS) indicates a 1 year survival of 83%, a 5 year survival of 70% and an 8 year survival of 61% (91). Survival is best in patients who are at home at the time of transplant compared to those who are in the hospital or in the ICU. A great advance in liver transplantation has been the improvement in immunosuppressive regimens so that allograft loss from rejection is now relatively rare (92,93).
The major issues that remain in the care of the patient post liver transplantation are recurrent disease in the transplant, particularly HCV, and longterm consequences of immunosuppressive agents such as hypertension, hyperlipidemia and renal disease.
Recent advances and future directions
Molecular pathology of hepatic fibrosis and cirrhosis
The scar tissue in cirrhosis is composed of a complex assembly of different extracellular matrix (ECM) molecules, comprising the fibril forming interstitial collagens type I and III, basement membrane collagen type IV, noncollagenous glycoproteins like fibronectin and laminin, elastic fibers and glycosaminoglycans and proteoglycans among others (94). Toxins, viruses, cholestasis, or hypoxia can trigger a wound healing reaction termed fibrogenesis, i.e., the excess synthesis and deposition of ECM. Initially, fibrogenesis is counterbalanced by removal of excess ECM by proteolytic enzymes, such as certain matrix metalloproteinases (MMPs) (95).
Chronic damage usually favours fibrogenesis over fibrolysis, with an upregulation of tissue inhibitors of MMPs (TIMPs) (79). The major hepatic ECM producing cells are myofibroblasts that either derive from activated hepatic stellate cells (HSC) or perivascular fibroblasts (96-98). Myofibroblast activation is mainly driven via fibrogenic cytokines and growth factors that are released by activated macrophages (Kupffer cells), other inflammatory cells and bile duct epithelia (Figure 2). The most prominent profibrogenic cytokine is transforming growth factor β which suppresses inflammation, but drives fibrogenic gene expression in these Myofibroblasts (96,98,99).
Genetic predisposition for cirrhosis
The variable rates of development of cirrhosis amongst individuals with similar risk factors such as HCV or alcohol abuse had long been unexplained. Recently, a growing number of functional genetic polymorphisms that likely increase the risk of fibrosis progression has been described. Implicated genes encode cytokines/chemokines and their receptors (100,101), molecules involved in fibrogenesis or fibrolysis (102), blood coagulation (103), antigen presentation (104), iron uptake (105), oxidative and antioxidative metabolism (106), detoxification (107) and polygenetic traits linked to the metabolic syndrome and NASH. In a recent gene association study 1,609 out of 24,882 single nucleotide polymorphisms (SNPs) were found to be associated with fibrosis progression in chronic hepatitis C, with the DDX5 gene having a high positive predictive value (108). Together with established extrinsic risk factors like excess alcohol consumption, obesity or advanced age these SNPs will allow the establishment of risk profiles for the individual patient (109).
Feasibility of pharmacological reversal of cirrhosis
The observations that even cirrhosis can regress once the fibrogenic trigger is eliminated (5,6,59,60,69-71,110) can be explained by the dynamic processes of fibrogenesis and fibrolysis even in cirrhosis (6). While the central role of activated HSC (myofibroblasts) in fibrogenesis is unchallenged, other cells contribute. Thus macrophages/Kupffer cells retarded progression in early but promoted progression in advanced fibrosis (111). Furthermore, regression from macro- to micronodular cirrhosis and possible cirrhosis reversal depends on the degree of ECM crosslinking, which is catalyzed by enzymes such as tissue
transglutaminase (112). The rapid progress in understanding the molecular mechanisms that lead to cirrhosis or its reversal have spawned the development of antifibrotic drugs. We can classify the therapeutic approaches to reversal of fibrosis as primary and secondary. Primary approaches focus on treatment of the underlying disease such as HBV and HCV that have convincingly been shown to result in regression of (compensated) cirrhosis (59,60,72). The secondary approach is to develop pharmacotherapy that is directly focused on the mechanism of fibrogenesis, intrinsic antifibrotic drugs, irrespective of the etiology of the liver disease.
The major obstacle to antifibrotic drug development has been the difficulty in defining validated endpoints for clinical trials. The combination of a slowly evolving disease (years to decades) and an established endpoint (liver biopsy) which has limited sensitivity and significant sampling variability represents a stumbling block for study design. In particular, without short term surrogate markers for liver fibrosis, exploratory studies are hampered by the need for significant sample size and high risk of failure.
Noninvasive markers of fibrogenesis and fibrolysis
Non-invasive serological markers to cross-sectionally stage liver fibrosis (113-123) have been extensively reviewed (124-126). Although showing potential, particularly for the diagnosis of cirrhosis, none meet the criteria for an ideal surrogate fibrosis marker (Table 8). A problem is the heterogeneity of liver diseases, with different stages being present in different areas of the liver, particularly between stages 1 to 3. These markers either reflect hepatic function (113-119) or turnover of the ECM (120-123) (Table 9). Combinations have been developed since no single biomarker has adequate sensitivity and specificity. Unfortunately, current ECM-derived serum markers correlate mainly with fibrosis stage, and to a lesser degree with fibrogenesis. We consider the performance of the majority of these biomarkers to be similar with a diagnostic accuracy approaching 80% for the differentiation between mild fibrosis (Metavir F0/1) and moderate to severe fibrosis (F2-4). However, the performance is consistently better at both spectrums of disease from no fibrosis to cirrhosis, and importantly, for predicting cirrhosis.
Differentiation of fibrosis stage F0-1 from F2-4 by serum markers and fibroscan
Hepatic elasticity measurement (Fibroscan) (42,126,127) in combination with these serum indices may yield a better prediction of histological fibrosis than either test alone (126), and a recent study showed that Fibroscan was superior to Fibrotest in hepatitis C patients with persistently normal or low transaminases (127).
Several of these tests are now available for use in clinical practice and there is a clinical role for surrogate fibrosis markers. A simple algorithm for using biomarkers is given in Figure 2. The major focus for research is to identify new biomarkers that allow assessment of the dynamic processes of fibrogenesis and fibrolysis, in order to monitor the effect of antifibrotic therapies in patients. This may be achievable with serum proteomics or glycomics (128, 129), or novel imaging techniques for sensitive assessment of fibrogenesis representing the whole liver. Such techniques could be based on CT or MRI imaging with the use of contrast media that target activated HSC. Their validation likely requires parallel analysis of the liver transcriptome of patients with slow or rapid fibrosis progression (130), an approach that requires invasive sampling of liver tissue.
Pharmacological and cellular reversal of hepatic fibrosis and cirrhosis
Numerous agents with proven direct and indirect antifibrotic effects in experimental animals would merit clinical testing (98,131-134), and efficient reversal therapies likely require antifibrotic drug combinations (Table 10, Figure 4). Of note, many potential antifibrotics possess a reasonable safety profile, while their long-term safety in cirrhotic patients has to be proven. However, optimization of such treatment heavily relies on the availability of sensitive non-invasive markers or techniques that allow their rapid testing in low numbers of patients.
Antifibrotic approaches and candidates for combination therapy
In order to achieve quick restitution of the functional parenchymal mass in concert with reversal of cirrhosis, the combination of antifibrotic therapy and hepatocyte renewal is attractive (135-137). Thus hepatocyte transplantation improved liver function (138,139) and ameliorated or even reversed advanced fibrosis (140,141). Hepatocyte engraftment was increased by oxidative preconditioning and HSC activation (142-143), and infusion of HGF, a potent hepatocyte mitogen, improved liver function (144). The isolation and in vitro expansion of hepatocyte stem or progenitor cells for cell transplantation may hold promise for an unlimited donor pool (145,146).
Reports that infusion of bone marrow stem cells replenished hepatocytes, either by hepatocytic transdifferentiation (147), fusion with hepatocytes (148,149), or indirectly by hepatotrophic growth factors released from stem cells engrafted in the hepatic vasculature (150) sparked much enthusiasm. However, efficiency of stem or progenitor cell engraftment is generally low (151) and the manipulations that are currently needed to allow for sufficient engraftment in humans would incur great risks for patients with cirrhosis and liver failure, necessitating considerable refinement before this techniques can be applied to patients. Similarly, the observation that genetic restitution of telomerase, an enzyme that abrogates cellular ageing by preventing chromosomal telomer shortening, can accelerate hepatic regeneration and ameliorate experimental liver fibrosis has evoked much interest (152). However, increased telomerase activity also favours hepatocarcinogenesis which dampens the enthusiasm for this approach (153).
Summary
Many advances have occurred in the clinical care of patients with cirrhosis and the complications of end stage liver disease. The majority of these have focused on treatment of the underlying cause of cirrhosis and management of complications of portal hypertension. The next 10 years may see us focus on the primary prevention and treatment of cirrhosis. Examples are the use of non-invasive tests to screen for earlier stages of fibrosis and to monitor antifibrotic drug effects, and pharmacological targeting of fibrogenesis pathways. Stem cell or hepatocyte transplantation aiming at reconstitution of liver function may become a clinical reality. Continued basic and clinical research is critical to be able to finally remove cirrhosis as an irreversible condition and a major contributor to morbidity and mortality in our patients.
Acknowledgements
We thank the Espinosa Liver Fibrosis Fund at Beth Israel Deaconess Medical Center and the LIFER Foundation, Boston, MA, for research support.